
Moreover, the "8D" process guides the quality team through every step of CAPA implementation, from identification of the problem through corrective action. The MasterControl clinical laboratory quality assurance and CAPA system interconnects different quality subsystems and tracks incidents that can escalate into a corrective action.

This ensures that any deviation or nonconformity from the standard is immediately reported. Quality assurance is a major part of testing, analyzing and observing processes in the system. MasterControl's validation tools and services dramatically reduce the time involved in validating a system, reducing the risk of project implementation and making it easier to validate software upgrades, all of which help contribute to a lower cost of validation. Organizations can either automate or manually validate their software solutions based on their risk assessment model. The entire validation solution is comprised of a combination of products and services that address different levels of validation needs. In order to help companies attain FDA compliance, MasterControl’s clinical laboratory quality assurance software provides validation tools and services.
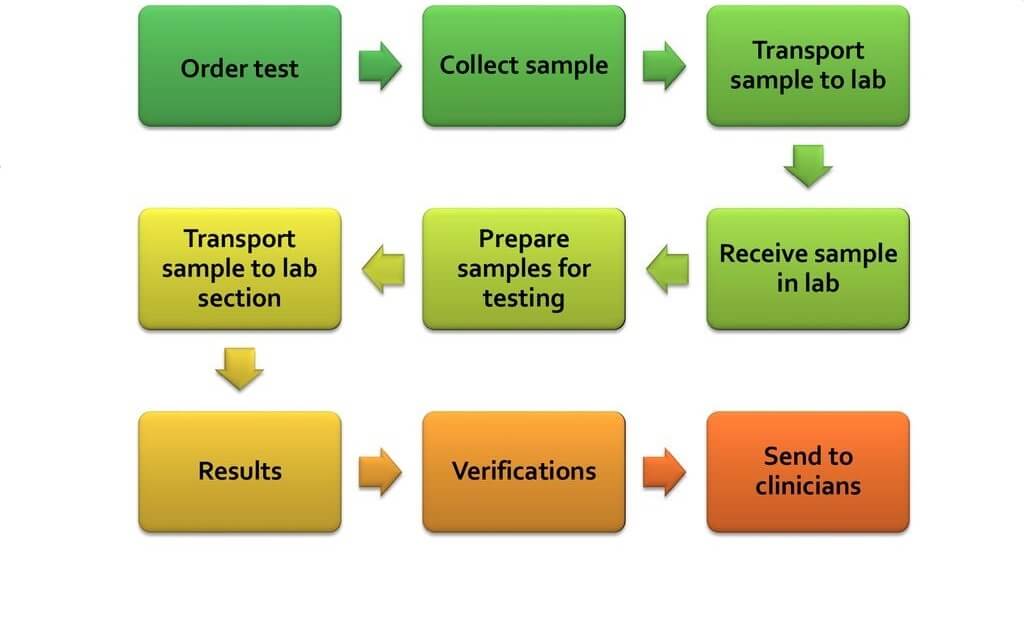

MasterControl is the leading pioneer in providing web-based solutions to companies that need to streamline their business processes and operations with a single web-based platform.Ĭlinical laboratory quality assurance software automates, streamlines, and effectively manages document control, change control, training control, audit management, corrective/preventive action (CAPA), customer complaints, and other quality processes. The aim of the MasterControl software is to integrate quality process management and to enable online cross communication between company departments.


 0 kommentar(er)
0 kommentar(er)
